Prepare a SOP on document control
Version 1 - Last updated 23rd July 2025
When prepared the standard operating procedure (SOP) should address the following points:
Access (protection and retrieval)
- Define access levels:
- View only for general staff.
- Edit/upload for authorised personnel, such as chemical responsible team or person.
- Designate a central authority to control all documents (preferably the Chemical Responsible Person/Team).
- Describe the workflow and procedures for managing chemical management system-related documents.
Location (retention)
- Ensure easy access for relevant staff.
- General documents (e.g., SDS or chemical policy) should be available to all staff.
- Department-specific documents (e.g., training records for HR, brand RSL documents for QC lab) should be accessible to relevant departments.
- Store documents as:
- Hard copies in files.
- Soft copies on the company server or open-source platforms (e.g., Google Drive).
- Assign access permissions for "view only" or "view and edit".
- Communicate the location(s) of all documents and records to the staff.
Review (updates and removal)
- Implement a regular review process.
- Update obsolete documents with new ones.
- Delete or destroy documents that are no longer valid.
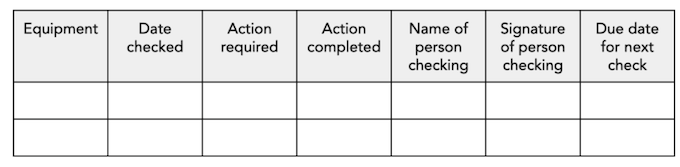
Following is a template which can be used to maintain a summary of revisions made in the chemical management system: